Research Highlights
Monolayer Fullerene Networks as Photocatalysts for Overall Water Splitting
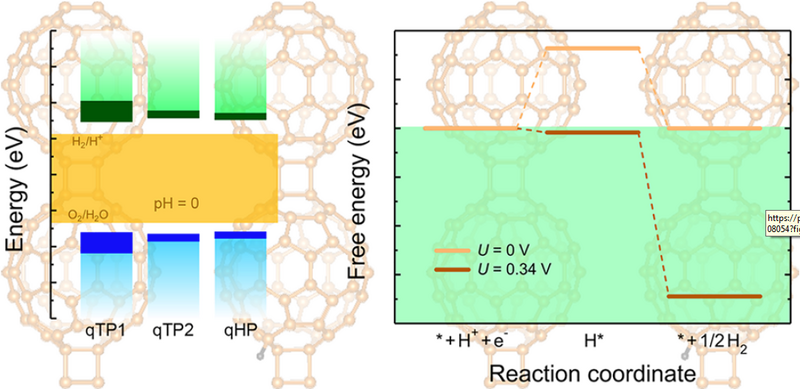
Photocatalytic water splitting can produce hydrogen in an environmentally friendly way and provide alternative energy sources to reduce global carbon emissions. Recently, monolayer fullerene networks have been successfully synthesized, offering new material candidates for photocatalysis because of their large surface area with abundant active sites, feasibility to be combined with other 2D materials to form heterojunctions, and the C60 cages for potential hydrogen storage. However, efficient photocatalysts need a combination of a suitable band gap and appropriate positions of the band edges with sufficient driving force for water splitting. In this study, I employ semilocal density functional theory and hybrid functional calculations to investigate the electronic structures of monolayer fullerene networks. I find that only the weakly screened hybrid functional, combined with time-dependent Hartree-Fock calculations to include the exciton binding energy, can reproduce the experimentally obtained optical band gap of monolayer C60. All the phases of monolayer fullerene networks have suitable band gaps with high carrier mobility and appropriate band edges to thermodynamically drive overall water splitting. In addition, the optical properties of monolayer C60 are studied, and different phases of fullerene networks exhibit distinct absorption and recombination behavior, providing unique advantages either as an electron acceptor or as an electron donor in photocatalysis.
Monolayer Fullerene Networks as Photocatalysts for Overall Water Splitting
Journal of the American Chemical Society
144 (43),
19921
(2022)