Research Highlights
Understanding the growth of silicon carbide crystals
Silicon carbide (SiC) consists of layers stacked on top of each other, with each layer being able to take one of two possible orientations. When all layers take the same orientation, the result is a cubic crystal, but there are many other possible forms.
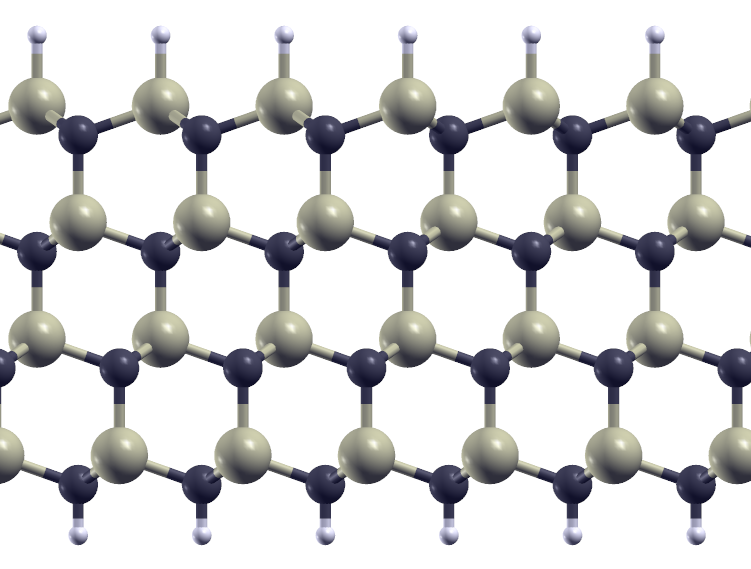
Very thin cubic SiC crystal, with the surfaces terminated by hydrogen. The top, silicon, surface has its orientation reversed. The reversal is clearly seen if one tracks the long, near-horizontal, bonds in each layer.
SiC has applications as a high-temperature semiconductor, being able to operate even when glowing dull red at 650°C, whereas silicon-based devices cannot be used above 150°C. However, the different stacking sequencies have different electronic properties, so it is important to be able to grow the desired form repeatably. An understanding of why SiC grows in the forms it does will help to control the growth.
Surprisingly SiC does not always grow in its lowest energy form. Indeed, it has been found that different forms will grow on different faces of a crystal under the same conditions.
The calculations here suggests that if one considers the energy of a single layer at the surface, then on one side of a SiC crystal it prefers to be in the cubic orientation, and on the other, it wishes to break a cubic stacking sequence. Once such a layer has formed and has been covered by further layers, it will be hard for it to transform into the other orientation. The stacking sequence will be frozen in, regardless of whether it is the lowest energy for the bulk crystal.
For further details see
Energetics of stacking boundaries on the {0001} surfaces of silicon carbide
J. Phys. - Condens. Mat.
9
8213 - 8220
(1997)