


Next: 2.3 Identical particles
Up: 2. Many-body Quantum Mechanics
Previous: 2.1 Principles of quantum
Contents
2.2 The Born-Oppenheimer approximation
The forces on both electrons and nuclei due to their electric charge are of
the same order of magnitude, and so the changes which occur in their momenta
as a result of these forces must also be the same. One might, therefore,
assume that the actual momenta of the electrons and nuclei were of similar
magnitude. In this case, since the nuclei are so much more massive than the
electrons, they must accordingly have much smaller velocities. Thus it is
plausible that on the typical time-scale of the nuclear motion, the electrons
will very rapidly relax to the instantaneous ground-state configuration, so
that in solving the time-independent Schrödinger equation resulting from the
Hamiltonian in equation 2.12, we can assume that the nuclei are
stationary and solve for the electronic ground-state first, and then
calculate the energy of the system in that configuration and solve for the
nuclear motion. This separation of electronic and nuclear motion is known
as the Born-Oppenheimer approximation [6].
Following Ziman [7], we assume the following form of an
eigenfunction for the Hamiltonian (2.12):
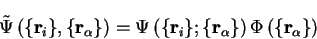 |
(2.18) |
and require that
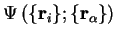 (which is a wave-function only of the
(which is a wave-function only of the
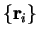 with the
with the
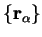 as parameters)
satisfies the time-independent Schrödinger equation for the electrons in a
static array of nuclei:
as parameters)
satisfies the time-independent Schrödinger equation for the electrons in a
static array of nuclei:
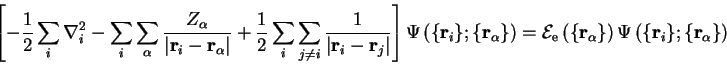 |
(2.19) |
in which the dependence of the eigenvalues
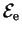 on the
nuclear positions is acknowledged. Applying the full Hamiltonian
(2.12) to the whole wave-function:
on the
nuclear positions is acknowledged. Applying the full Hamiltonian
(2.12) to the whole wave-function:
The energy
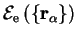 is
called the adiabatic contribution of the electrons to the energy of the
system. The remaining non-adiabatic terms contribute very little to the
energy, which can be demonstrated using time-independent perturbation theory
[8]. The first order correction arising from
the first non-adiabatic term in the last line of equation
2.20 is of the form:
is
called the adiabatic contribution of the electrons to the energy of the
system. The remaining non-adiabatic terms contribute very little to the
energy, which can be demonstrated using time-independent perturbation theory
[8]. The first order correction arising from
the first non-adiabatic term in the last line of equation
2.20 is of the form:
and the term in square brackets can be rewritten
since the normalisation of the electronic wave-function does
not change when the nuclei move, so that the first order contribution vanishes.
The second-order shift due to this term does not vanish and gives rise to
transitions between electronic states as the ions move, otherwise known as the
electron-phonon interaction, which will modify the energy.
The second non-adiabatic term in the final term of equation 2.20
will be largest when the electrons labelled 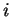 are tightly bound to the nuclei
labelled
are tightly bound to the nuclei
labelled 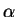 in which case
in which case
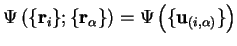 where
where
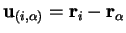 and the
first order correction from this term is
and the
first order correction from this term is
and this quantity is of the order of the electronic kinetic energy
multiplied by the ratio of the electron and nuclear masses,
typically a factor of the order of 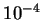 or
or 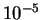 , so that
the contributions from this term to all orders can be neglected.
, so that
the contributions from this term to all orders can be neglected.
We therefore neglect the non-adiabatic terms and note that equation 2.20 is
satisfied if
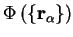 obeys a
Schrödinger equation of the form
obeys a
Schrödinger equation of the form
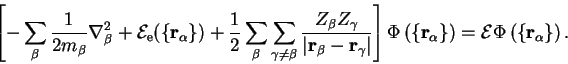 |
(2.23) |
This adiabatic principle is crucial because it allows us to separate the nuclear
and electronic motion, leaving a residual electron-phonon interaction.
From this point on it is assumed that the electrons respond instantaneously
to the nuclear motion and always occupy the ground-state of that nuclear
configuration. Varying the nuclear positions maps out a multi-dimensional
ground-state potential energy surface, and the motion of the nuclei in
this potential can then be solved. In practice Newtonian mechanics generally
suffices for this part of the problem2.7, and relaxation of the nuclear positions
to the minimum-energy configuration or
molecular dynamics [11,12] can be performed.
These aspects go
beyond the scope of this dissertation so that from now on it is
assumed that a system with a fixed nuclear configuration is to be treated,
so that the electronic energy
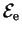 is a constant and the
electronic wave-function
is a constant and the
electronic wave-function
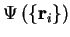 obeys the Schrödinger equation
2.19. The dependence of the electronic wave-function on the
nuclear positions
obeys the Schrödinger equation
2.19. The dependence of the electronic wave-function on the
nuclear positions
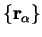 is now suppressed.
is now suppressed.



Next: 2.3 Identical particles
Up: 2. Many-body Quantum Mechanics
Previous: 2.1 Principles of quantum
Contents
Peter Haynes
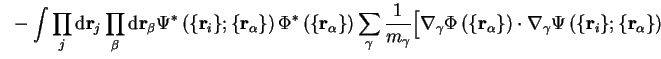
![$\displaystyle - \sum_{\gamma} \int \prod_{\beta} {\mathrm d}{\bf r}_{\beta}
\Ph...
...la}_{\gamma} \Psi \left( \{ {\bf r}_i \} ; \{ {\bf r}_\alpha \} \right)
\right]$](img70.gif)
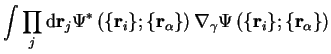
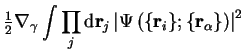
![]() are tightly bound to the nuclei
labelled
are tightly bound to the nuclei
labelled ![]() in which case
in which case
![]() where
where
![]() and the
first order correction from this term is
and the
first order correction from this term is
![$\displaystyle ~- \int \prod_j {\mathrm d}{\bf r}_j \prod_{\beta}
{\mathrm d}{\b...
...\right) \nabla_{\gamma}^2
\Psi \left( \{ {\bf u}_{(i,\alpha)} \} \right) \Bigr]$](img77.gif)
![$\displaystyle - \sum_{\gamma} \frac{1}{2 m_{\gamma}} \left[ \int \prod_{\beta}
...
...right) \nabla_{\gamma}^2
\Psi \left( \{ {\bf u}_{(i,\alpha)} \} \right) \right]$](img78.gif)
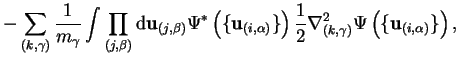
![]() obeys a
Schrödinger equation of the form
obeys a
Schrödinger equation of the form
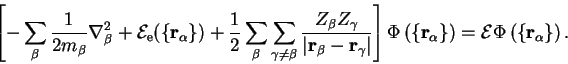
![]() is a constant and the
electronic wave-function
is a constant and the
electronic wave-function
![]() obeys the Schrödinger equation
2.19. The dependence of the electronic wave-function on the
nuclear positions
obeys the Schrödinger equation
2.19. The dependence of the electronic wave-function on the
nuclear positions
![]() is now suppressed.
is now suppressed.