The method of variance minimisation was first applied to quantum mechanical problems in the 1930's. It was first used in QMC calculations by Coldwell [61], and some of the most impressive QMC applications have been by Umrigar and coworkers [22, 30]. Umrigar developed the variance minimisation technique [22] to calculate wavefunctions for use in VMC and DMC calculations on the Be atom. He took the standard atomic trial wavefunction
![]()
where ![]() is equal to 1/2 for antiparallel-spin electrons and 1/4
for parallel-spin electrons, to satisfy the cusp condition.
He then optimised the value of the parameter
b using variance minimisation.
is equal to 1/2 for antiparallel-spin electrons and 1/4
for parallel-spin electrons, to satisfy the cusp condition.
He then optimised the value of the parameter
b using variance minimisation.
He then attempted to generalise the Jastrow part of the wavefunction to take account of the individual positions of the electrons as well as the electron-electron separation.
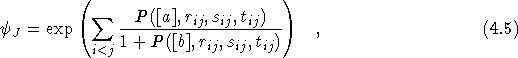
where
![]() and
P is a complete
and
P is a complete ![]() order polynomial in r, s and t with
sets of coefficients [a] and [b]. The sets of coefficients [a]
and [b] were then optimised using the same variance minimisation
technique. The error in the expectation value of the energy was
reduced from 0.001 to 0.000003 Hartrees by the optimisation process.
order polynomial in r, s and t with
sets of coefficients [a] and [b]. The sets of coefficients [a]
and [b] were then optimised using the same variance minimisation
technique. The error in the expectation value of the energy was
reduced from 0.001 to 0.000003 Hartrees by the optimisation process.
Recently, Umrigar and Filippi[62] have extended their work to study first row diatomic molecules with QMC. They have used multiconfigurational wavefunctions and used the variance minimisation method to not only optimise the Jastrow and functions but also to optimise the wavefunctions with respect to some variational parameters present in the one-electron wavefunctions that make up the Slater determinants.
Mitas and Martin have also optimised wavefunctions for use in atomic
QMC calculations [41]. They also chose a two-body correlation
function that depends on the electron-electron separation and an
additional electron-ion term, ![]()
where
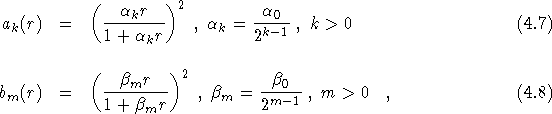
and ![]() is the separation between the
is the separation between the ![]() electron and the
electron and the
![]() ion, and
ion, and ![]() . The largest range of k,l,m
used was from 0 to 5 but only a subset of values were actually used.
. The largest range of k,l,m
used was from 0 to 5 but only a subset of values were actually used.
When performing VMC calculations on atomic and molecular nitrogen
[63], Mitas and Martin used 21 variational parameters in
![]() . These were optimised using the
Umrigar minimisation of variance technique.
. These were optimised using the
Umrigar minimisation of variance technique.
Mitas and Martin have also begun optimising wavefunctions for use in
QMC calculations on solids. They used the same correlation
function, Eq.(![]() ) from the nitrogen atom calculations, but
with only 6 optimised parameters, to perform calculations on solid
nitrogen.
) from the nitrogen atom calculations, but
with only 6 optimised parameters, to perform calculations on solid
nitrogen.
A few QMC trial/guiding wavefunctions have also been generated by methods other than the Umrigar minimisation of variance technique [22]. Tanaka [64] has performed VMC calculations on the cohesive energy of NiO using wavefunctions generated by minimising the total energy of the wavefunction. He used a trial wavefunction where the Jastrow factor took its standard form
![]()
and the Chi function was expressed as a sum of Gaussians
![]()
where ![]() is the electron position and
is the electron position and ![]() are the positions of
the ionic cores.
are the positions of
the ionic cores.